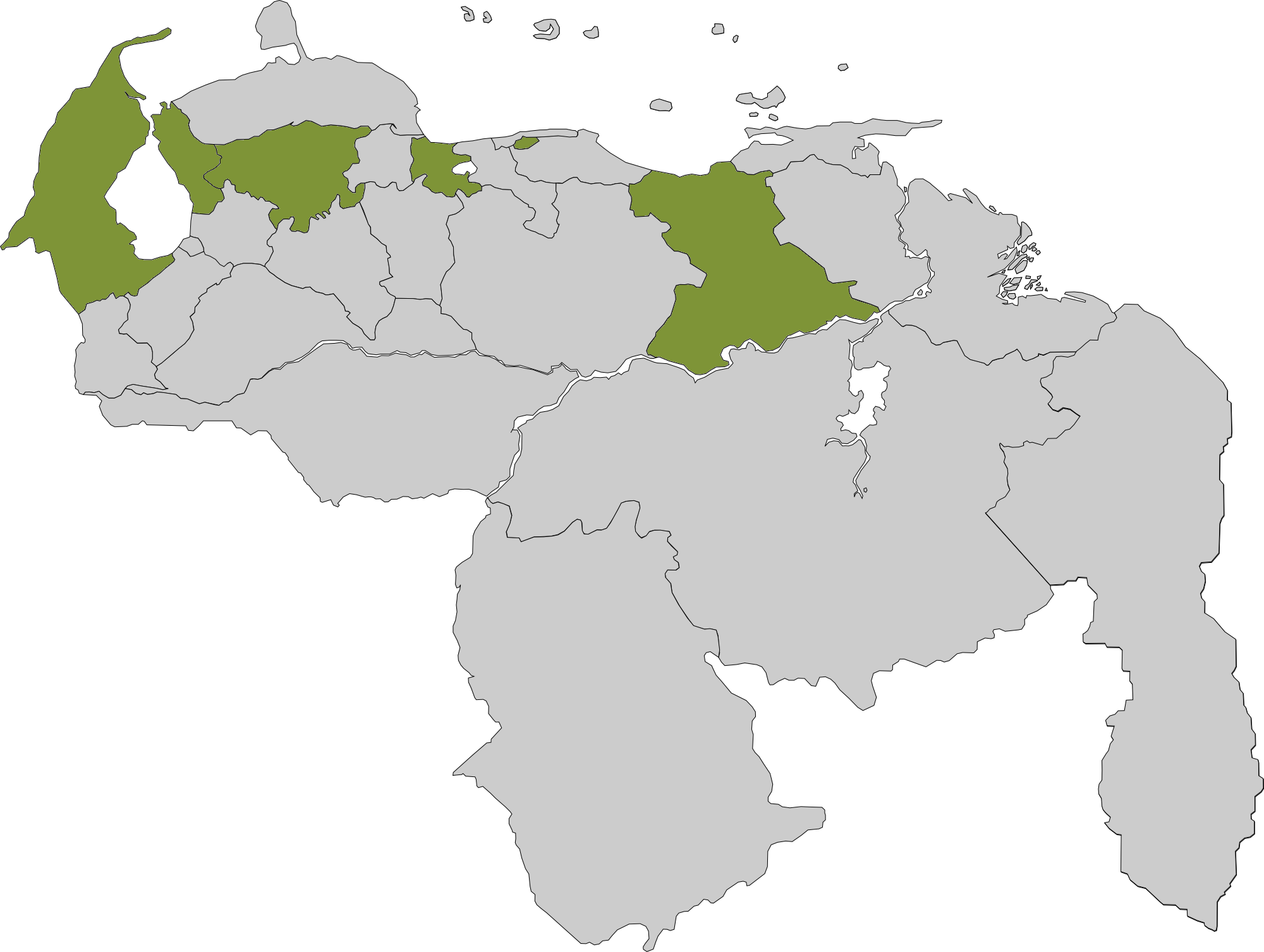
Travieso Evans Arria & Rengel fue fundada en 1929, y se ha mantenido por décadas como una de las principales firmas de Venezuela. Actualmente, cuenta con oficinas propias en las ciudades de Caracas, Maracaibo, Valencia, Barquisimeto y Puerto La Cruz.
Más de 90 Años de Experiencia
Nuestra Filosofía
Prestar servicios legales integrales de alta calidad y eficiencia, cumpliendo los estándares internacionales, manteniendo el más alto nivel de ética profesional con un profundo compromiso de responsabilidad social.
Quiénes Somos
Nuestro equipo cuenta con abogados formados en las mejores Escuelas de Derecho del país; y con estudios de Postgrado en Venezuela y distintas Universidades extranjeras. Nos encontramos siempre a disposición para acompañar a los clientes en todas sus necesidades y proyectos, con experiencia y una visión innovadora.

Nuestra Filosofía
ÁREAS DE PRÁCTICA
Podrás tener mayor información al pulsar el área de tu interés.
Nuestros Miembros
Socios
Asociados
Oficinas a tu alcance
Nuestras Sedes
Caracas
Teléfono (+58 212) 918.3333
Valencia
Teléfonos (+58 241) 825.6456 / 826.2821
Puerto La Cruz
Teléfonos (+58 281) 286.8683 / 286.7898
Barquisimeto
Teléfonos (+58 251) 233.7537 / 233.6552
Maracaibo
Teléfonos (+58 261) 792.0261 / 793.5754
Caracas

La Firma tiene su sede principal en la ciudad de Caracas desde su fundación en el año 1929, desde la cual se apoya a través de sus diferentes sucursales a nivel nacional. Nuestra oficina en Caracas nos permite prestar servicios a toda el Área Metropolitana, Estado Miranda y Estado Vargas.
Dirección
Edificio Mene Grande, Piso 14, Avenida Francisco de Miranda, Los Palos Grandes, Caracas. D.C. – Venezuela ZP 1060.
Cómo llegar aquí
Teléfono
(+58 212) 918.3333
Puerto La Cruz

En el año 2003 se inauguró la Oficina de Puerto La Cruz, con el objetivo de prestar servicios a los clientes en el Oriente del País. Desde esta oficina prestamos servicios regularmente en ciudades como Barcelona, Lechería, Puerto La Cruz, Guanta, Anaco, Cantaura, El Tigre, El Tigrito o San José de Guanipa, San Tomé, Puerto Píritu (Complejo Petroquímico de Jose), Cumaná, Ciudad Bolívar, Puerto Ordaz y Maturín.
Dirección
Torre Banco Venezolano de Crédito, Piso 6, Ofic. 6J, Av. Intercomunal Jorge Rodríguez, Sector Las Garzas, Lecherías, Edo. Anzoátegui – Venezuela ZP 6016.
Cómo llegar aquí
Teléfonos
(+58 281) 286.8683 / 286.7898
Valencia

Travieso Evans Arria & Rengel abrió sus oficinas en la ciudad de Valencia en el año 2000, y desde hace casi dos décadas asiste a los clientes ubicados en las zonas industriales más importantes del centro del país y a lo largo de las principales ciudades de los Estados Carabobo, Aragua, Cojedes y Guárico.
Dirección
Torre Movilnet, Piso 7, Oficina 3, Avenida Paseo Cabriales, Valencia. Edo. Carabobo -Venezuela ZP 2001.
Cómo llegar aquí
Teléfonos
(+58 241) 825.6456 / 826.2821
Barquisimeto

En el año 2012, la Firma abrió una nueva oficina en la ciudad de Barquisimeto a los fines de expandir su cobertura territorial a los Estados Lara, Yaracuy, Portuguesa y Barinas.
Dirección
Centro Ejecutivo Yacambú, Piso 5, Ofic. 5-5, Carrera 19 entre Calles 22 y 23, Barquisimeto, Edo. Lara – Venezuela ZP 3001.
Cómo llegar aquí
Teléfonos
(+58 251) 233.7537 / 233.6552
Maracaibo

Desde el año 2007, Travieso Evans Arria & Rengel cuenta con su sede en la ciudad de Maracaibo con la finalidad de atender las necesidades de los clientes en el occidente del país, en las principales ciudades de los Estados Zulia, Falcón, Táchira, Mérida y Trujillo.
Dirección
Unicentro Virginia, Piso 2, Local 2-12, Avenida 3C con Calle 67, Maracaibo, Edo. Zulia – Venezuela ZP 4002.
Cómo llegar aquí
Teléfonos
(+58 261) 792.0261 / 793.5754
Puede escribirnos directamente a nuestro correo electrónico, donde nuestro equipo de profesionales podrá orientarle y asesorarle con cualquier duda o requerimiento.






























